Rapid Immunohistochemistry
the simplicity of H&E with the sensitivity and specificity of IHC

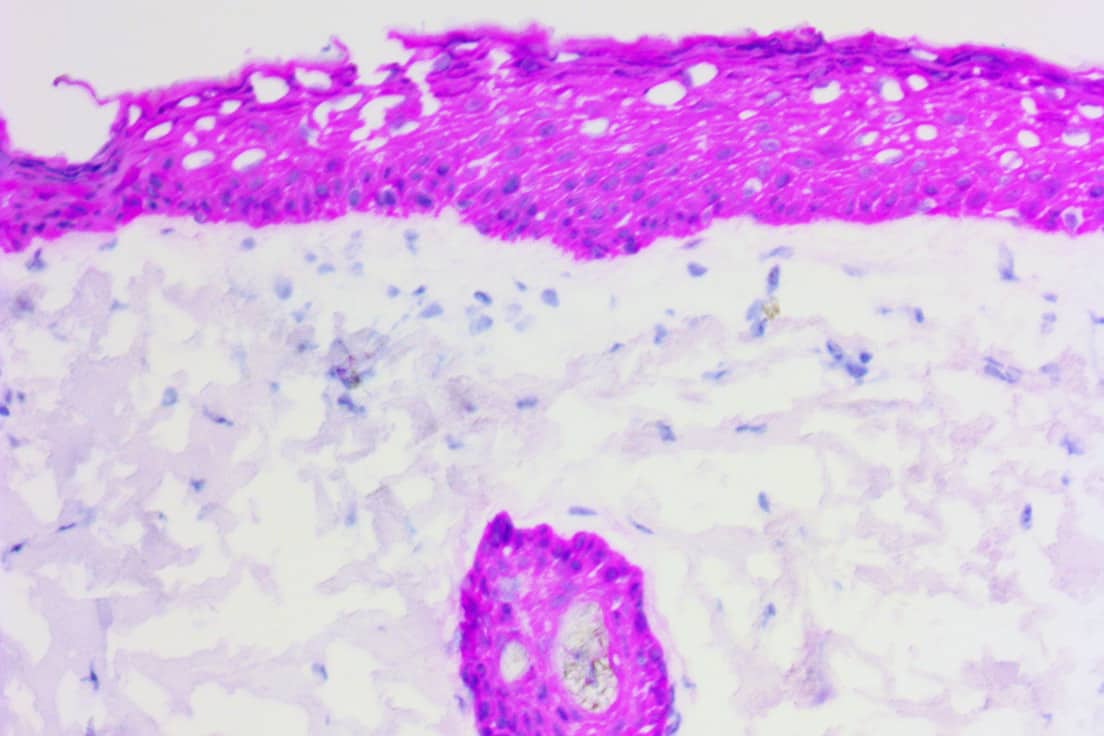
(ihcDirect Pan-CK 4Abs on frozen skin section with Gill 3 counterstain)
ihcDirect® assays deliver fast, simple and sensitive IHC results via direct conjugation or our primary antibodies to our proprietary polymerized horseradish peroxidase (polyHRP) detection system.

Get IHC results in as little as 10 minutes on frozen sections

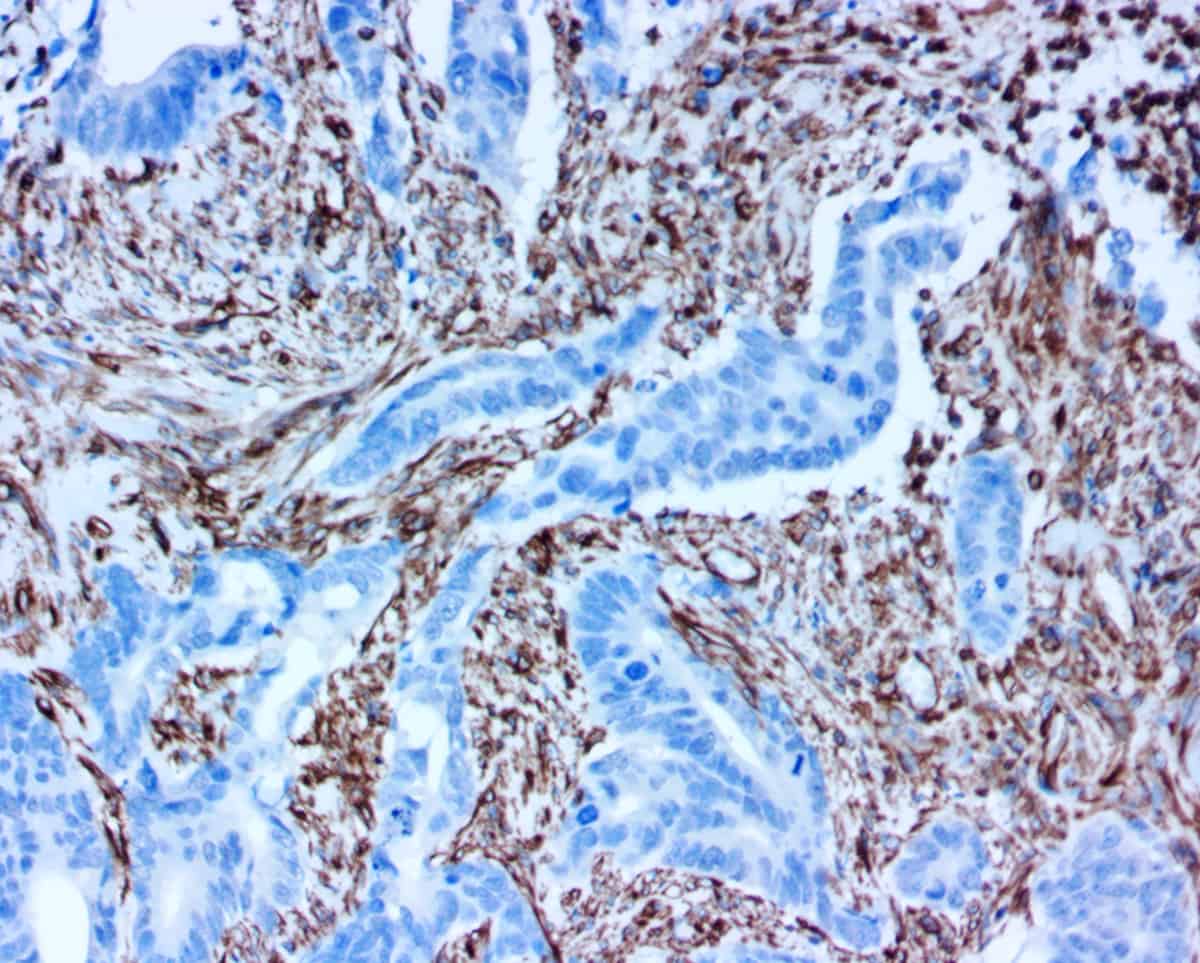
Better distinction between inflammation and cancer when H&E’s are difficult to read
Workflow benefits (save 40-60 min) when using FFPE sections and automated stainers
Explore all of our IHC assay kits and reagents
Resources to help you succeed
We have a comprehensive Literature Center with instructions for use, reference publications, and more to help you succeed while using Novodiax immunohistochemistry products. Our experts are also available to help answer questions and provide technical support remotely and in-person.
→ Literature Center → Contact SupportServices and co-development
Would you like a partner to help advance your research and product development? Our polyHRP technology is well-suited to a variety of innovative applications, such as converting therapeutic antibodies into “ready to use” companion diagnostics for clinical trials. We can also help with target validation and antibody profiling, custom and bulk assay kit development, and much more.
→ Learn More