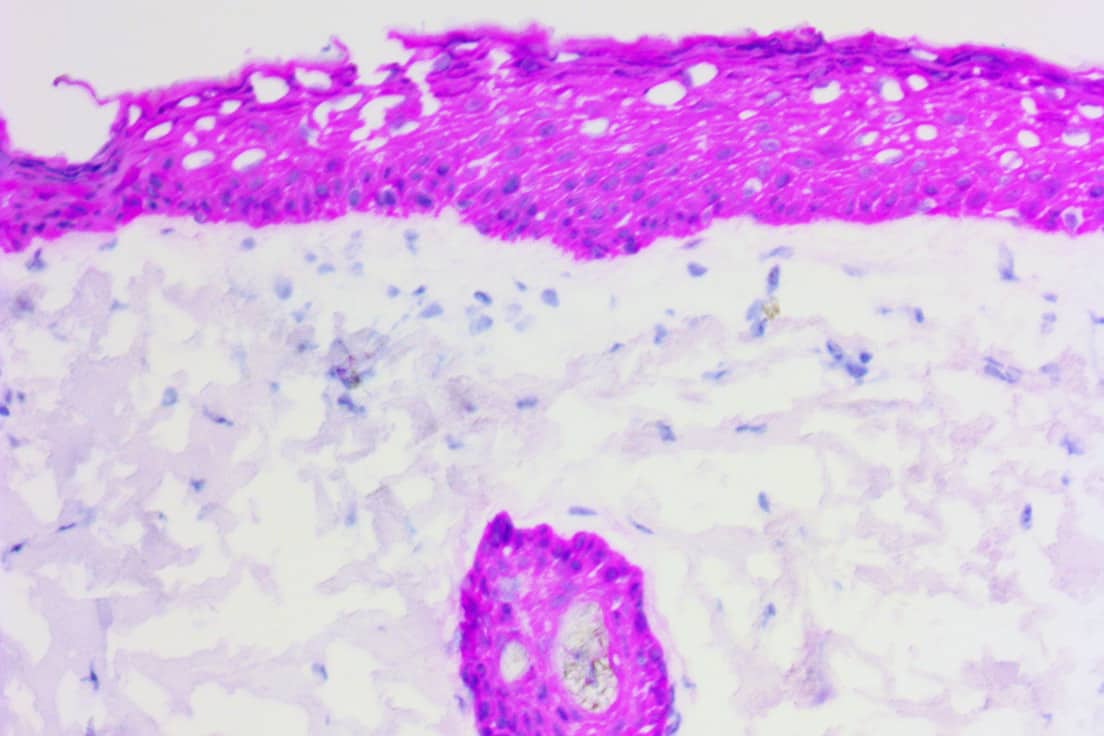
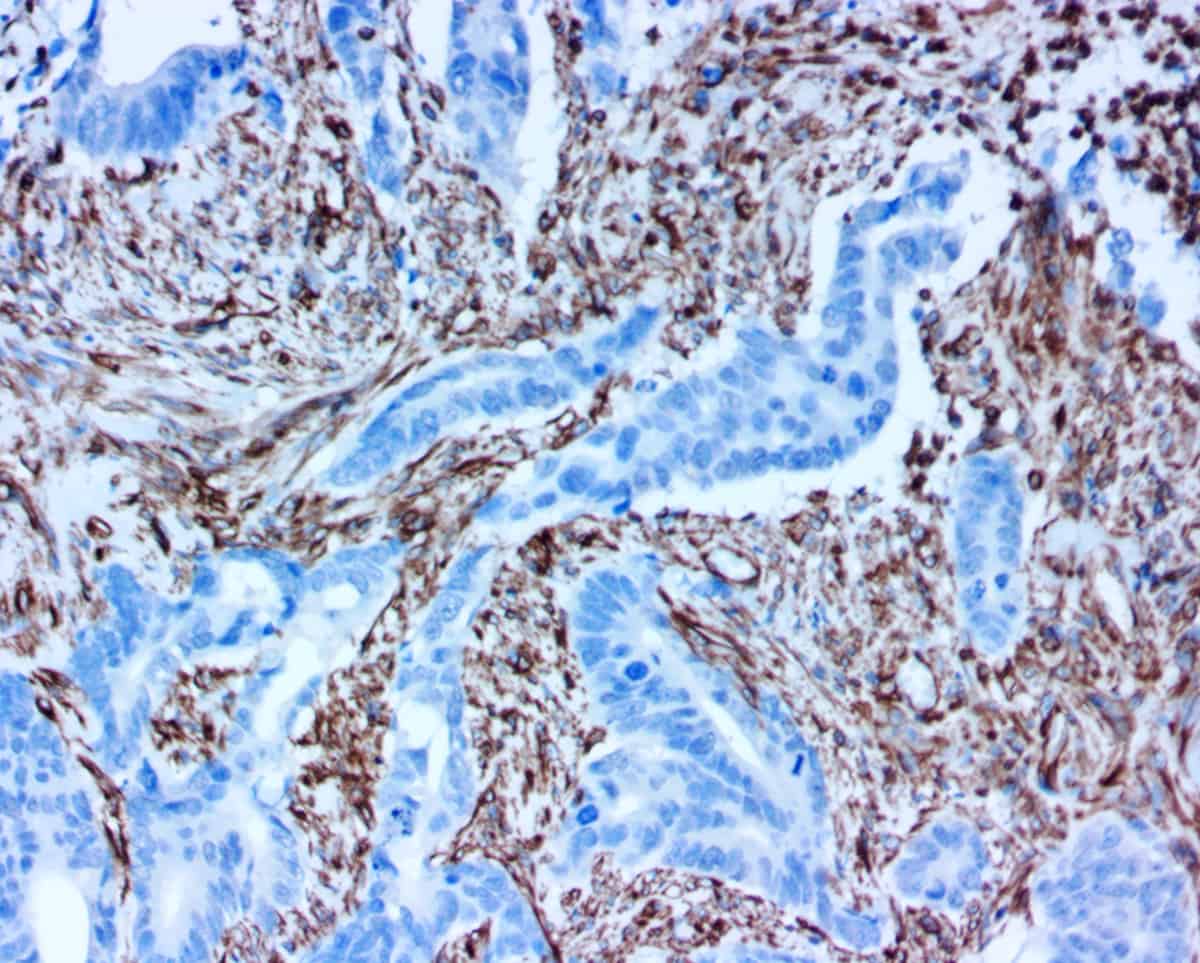
Troubleshooting Tips
Tissue Preparation: Cryotomy & Fixation
Not using positively charged slides can result in tissue loss.
Using positive charged slides or adhesion slides is required.Positively charged glass slides help negatively charged tissue adhere.
Alternatively, some users heat slides to ~50°C for a 30-seconds-to-1-minute, however heating slides can have other consequences like changes in cell morphology.
Incorrect fixative used.
Refer to the IFU to determine appropriate fixative; EtOH is often used in histology but is generally an undesirable fixative for antibody assays.
Tissue under-fixed.
Fixation is an essential part of tissue preparation for immunoassays.
Slides contain excessive fixative.
After fixation, allow slides to air dry for approx. 1 minute and rinse with 1X wash buffer before beginning protocol.
Fatty and/or other non-standard tissues can cause tissue loss.
Fatty tissues may not adhere as reliably to microscope slides. In some cases, fixing tissue slides via warming may help to prevent tissue loss. Users may also apply different cutting techniques and cryostat temperatures when cutting fatty, cartilaginous, bony, or other non-standard tissues to help them better adhere to the slide. Each set of tissues may require unique fixation protocols.
IHC Staining: Blocking, Antibody, & Chromogen
Pressure from rinsing with lab wash bottle is too strong.
Cut tips of lab wash bottles and rinse starting from the top of the slide downwards.
Another option is to dip slides via a slide rack into a wash tank or staining tub. Shake slides in wash solution for 30-45 seconds.
Loss of tissue in staining tub/wash tank/Coplin jar.
Use care / slow down the process when washing slides so as to not lose tissue during the wash dipping procedure. Charged slides can help to prevent loss of tissue.
Selection of Slides.
Select positively charged slides to help tissues to adhere to the slide.
Some users may also attempt to use non-charged slides but use heat to adhere the tissue.Although using heat for a non-charged slide may help the tissue adhere, we advise using caution, because the heating process may also cause undesirable changes to the tissue morphology.
Tissue Preparation: Cryotomy & Fixation
Tissue section was cut too thick.
Thickly cutting tissues could mean that vital and potentially diagnostic areas of the tissue may be under represented or missed. Optimal tissue thickness for ihcDirect IHC testing is considered to be ~4-6µm. Users should optimize and validate procedures for thickness.
Under-fixed tissue causing autolysis or cellular self-digestion which can occur when tissue is removed from the body and is improperly preserved or not preserved quickly enough.
Under-fixed tissues can potentially lead to primary antibody and chromogen binding to tissues in a non-specific manner to necrotic cells or cellular components. Users may want to extend fixation times to 2-3 minutes to compensate.
IHC Staining: Blocking, Antibody, & Chromogen
Incubation too short, not applied, or rinsed off- Unblocked endogenous tissue elements interact non-specifically with primary antibody and/or chromogen.
Ensure that blocking is done for recommended amount of time; Blocker should not be rinsed off before the antibody application and incubation. Once applied and incubated, users should tap off excess blocker and remove excess blocker from around tissue with Kim Wipe or towel.
Incorrect ihc Wash Buffer dilution.
Use graduated cylinder to dilute 10X ihc Wash Buffer to 1X = 1-part 10X buffer + 9-parts deionized or distilled water.
Store ihc Wash Buffer at room temperature. If buffer becomes cloudy or has particulates, allow the reagent to equilibrate to room temperature. Mix thoroughly prior to diluting wash buffer to 1X concentration.
Antibody incubation time is too long- unbound antibody in the solution can bind non-specifically to necrotic cells or cellular components within the tissue specimen.
Adjust antibody incubation time to that specified or validated by good quality lab procedures; Increase rinse time (DAB= using a wash bottle, 10-15 seconds per slide and across all slides) (Magenta= using a staining dish with a rack, actively shake slides for 30-45 seconds).
Incorrect DAB Working Solution concentration (within dilution microtube)- incorrect chromogen-to-diluent ratio, expired reagent, and/or evaporated chromogen or mixed working solution has become too concentrated from overexposure to air.
Make new batch of DAB.
With DAB Kit, prepare by mixing 1 drop (30µl) of chromogen per 1ml of diluent. With ihc DAB 1:1, prepare equal volumes of the chromogen to diluent.In either case, invert mixture in microtube several times to ensure successful mixing.
Chromogen incubation too long- Chromogen will bind nonspecifically.
Use validated incubation time; Decreased incubation time may be necessary in some lab environments; decrease incubation by 1 minute; Increase rinse time (DAB= using a wash bottle, 10-15 seconds per slide and across all slides) (Magenta= using a staining dish with a rack, actively shake slides for 30-45 seconds).
Incubation temperature too warm or too cold.
Run IHC between 21-30°C; use a calibrated thermometer to verify room and/or slide warmer temperatures. When using the ihc Slide Manager, measure the temperature at the surface of the device rather than at the surface of the slide warmer. Generally, the temperature setting on the ihc Slide Warmer is 34.5°C-35°C to achieve 30°C on the surface of the ihc Slide Manager.
Artifacts/Folds- Chromogen is trapped in folds/artifacts created during cryotomy.
Ensure blade is sharp, clean, and properly angled (~5°). Then resection block.
Use care when washing tissues on the slide from a spray bottle with nozzle. Gently rinse tissues on the slide with 1X wash buffer so the force from the spray does not flip the edge of the tissue over the top of neighboring tissue regions.
Tissue Preparation: Cryotomy & Fixation
Tissue section is too thin.
The ideal tissue thickness for IHC is 4-6µm; Thin tissue sections have the risk of breaking or yielding a weaker signal. Users should optimize and validate procedures for thickness.
Fatty and/or other non-standard tissues can cause tissue loss.
Fatty tissues do not adhere to microscope slides as well or as strongly as other tissues; warming tissue slides is recommended to prevent tissue loss; Users may need to use different cutting techniques and cryostat temperatures when cutting fatty, cartilaginous, bony, or other non-standard tissues. Each of these tissues may require unique fixation protocols.
Tissue at room temperature too long before freezing- Leaving fresh tissue without fixation or freezing can result in a loss of antibody binding sites (epitopes).
Once tissue is excised, embed tissue in OCT as soon as possible, then leave chuck in the cryostat until ready to cut slides; Recommended to cover tissue blocks with OCT if they cannot be processed immediately.
Sectioned tissue on slides at room temperature too long- loss of epitopes and/or cell morphology.
Have fixative near the cryostat and fix tissues immediately or within a short time after cutting.
Not using positively charged slides can result in tissue loss.
Positively charged glass slides help your negatively charged tissue adhere.
Under-fixation causing protein denaturation/degradation- after fixation, sectioned tissue on slides left at room temperature too long may dry out leading to loss of epitopes and/or cell morphology.
Fixation is an essential part of tissue prepare for immunoassays.
Based upon the experiences of hundreds of users, fixing tissues for 1-2 minutes appears to be the minimum amount of fixation required. Some users fix tissues for longer times depending upon which tissue they are processing.
After fixation, it is helpful to put slides into a staining dish or Coplin jar filled with 1X Wash Buffer (PBS-T) or a PBS solution until ready to start the IHC protocol. This keeps tissues moist.
Incorrect Fixative.
Refer to the IFU to determine appropriate fixative; EtOH is often used in histology but is generally an undesirable fixative for antibody assays.
Excess OCT remains on slides after rinsing with wash buffer- prevents antibodies from binding to antigen.
Two washes in warm PBS-T, heat two plastic coplin jars with 50mL of PBS/Triton to 45°C in the microwave and rinse the slide 5-10 secs in each jar.
IHC Staining: Blocking, Antibody, & Chromogen
Wash buffer stream pressure from rinsing tissues with lab wash bottle is too strong. Uncut nozzle tips of lab wash bottles can slice through tissues when directly applied to the tissue.
Cutting wash bottle nozzle tips and gently rinsing the tissue from the top of the slide downwards so the wash buffer streams over the tissue rather than directly on the tissue will prevent tissue loss. Another option is to use a staining dish with a rack and shake slides up and down in wash buffer.
Unlevel surface or entire tissue not covered- reagents may run to backside of slides or unevenly stain the tissue.
Remove excess fluids following wash or blocking steps to keep the backs of slides dry.Apply antibodies directly onto tissue and cover tissue fully with antibody, blocker or chromogens.Maintain slides on a level surface to prevent uneven staining. A solid lab bench or leveling tool such as a Bullseye Spirit Level can help to ensure there is a level surface.
Improper reagent storage and handling- Contamination, degradation of detection components. If the integrity of the detection components is compromised, proper binding can be negatively impacted and overall
staining diminished.
Ensure all reagents are stored appropriately based off their respective reagent package labels: A) Store 10X Wash Buffer at room temperature 15-30°C, even after an individual bottle has been opened. Store only 1X wash buffer after preparation. If wash buffer bottles were stored in the refrigerator and particulates appear, wait for the bottle to return to room temperature before mixing. Particulates generally disappear once the 10X bottle has equilibrated. For further instructions, see ihc Wash Buffer IFU. B) Take ihcDirect reagents out of refrigerator approximately 15 minutes before use to allow the reagents to equilibrate. C) Antibodies arrive prediluted and can settle when stored for extended periods of time. Before applying antibody to tissue, invert or gently swirl or shake the bottle 10-20 times.
Incubation temperature too cold. Colder lab temperatures can slow the antibody-antigen reaction and lead to light or no staining.
Run IHC tests between 21-30°C. To accommodate cooler temperatures, users may purchase an ihc Slide Warmer or extend incubation times to accommodate the lab environment;
Use a calibrated thermometer to help verify that room and/or slide warmer temperatures meet the minimum requirements during incubation.
Excess 1X Wash Buffer left on slide can cause dilution of chromogen.
Ensure excess 1X Wash Buffer is tapped off slide and excess fluids are removed from around the tissue and the back of the slide using a Kim Wipe or absorbent towel.
Incompatible enzyme-chromogen interaction.
Use Only HRP-compatible chromogens.
Tissue Preparation: Cryotomy & Fixation
Under-fixation causing protein denaturation/degradation- after fixation, sectioned tissue on slides left at room temperature too long may dry out leading to loss of epitopes and/or cell morphology.
Fixation is an essential part of tissue prepare for immunoassays.
Based upon the experiences of hundreds of users, fixing tissues for 1-2 minutes appears to be the minimum amount of fixation required. Some users fix tissues for longer times, e.g. 3 minutes or longer depending upon which tissue they are processing.
After fixation, it is helpful to put slides into a staining dish or Coplin jar filled with 1X Wash Buffer (PBS-T) or a PBS solution until ready to start the IHC protocol. This keeps tissues moist.
Incorrect Fixative- Use of other fixatives like alcohols may result in a lack of adequate protein preservation. These fixatives may also damage or alter target epitopes. If the target antigen/epitope is damaged or denatured, the antibody cannot successfully bind to the antigen.
Determine best fixative for tissue, generally Acetone or 10% Neutral Buffered Formalin. If signal is too weak, users may try to fix tissues for longer times (e.g. 2-3 minutes).
Insufficient fixative concentrations – as fixatives can become diluted with repeated uses or less effective over long periods of time.
Make sure to regularly refresh fixative containers used for fixing slides. At minimum, it is recommended to change every other week. Users wanting to maximize the use of their fixatives may have to extend fixation times to compensate for the diluted or less effective reagent.
IHC Staining: Blocking, Antibody, & Chromogen
Blocker Incubation too short (or none at all)- unblocked endogenous tissue elements interact non-specifically with primary antibody and/or chromogen.
Ensure that blocking reagents are fresh and the reagent is added to the tissues for the recommended amount of time.
Excess Blocker remaining on the slide can cause dilution of conjugated antibody.
Once Blocker is applied and incubated, users should tap off and remove the excess blocker from around tissue using a Kim Wipe or absorptive wipe to optimize the antibody-epitope binding.
Antibody incubation time is too short- minimal epitope-antibody binding; Antibody does not have enough time to locate and bind to target.
Use validated incubation time; Some physicians like to see a darker signal. To obtain a darker signal, users may experiment with longer incubation times (e.g. increase from 3 minutes to 4-5 minutes). Users should validate any increased incubation times to that specified or validated by good quality lab procedures.
Unlevel surface or entire tissue not covered- reagents may run to backside of slides or unevenly stain the tissue.
Remove excess fluids following wash or blocking steps to keep the backs of slides dry.Apply antibodies directly onto tissue and cover tissue fully with antibody, blocker or chromogens.Maintain slides on a level surface to prevent uneven staining. A solid lab bench or leveling tool such as a Bullseye Spirit Level can help to ensure there is a level surface.
Improper reagent storage and handling- Contamination, degradation of detection components. If the integrity of the detection components is compromised, proper binding can be negatively impacted and overall
staining diminished.
Ensure all reagents are stored appropriately based off their respective reagent package labels: A) Store 10X Wash Buffer at room temperature 15-30°C, even after an individual bottle has been opened. Store only 1X wash buffer after preparation. If wash buffer bottles were stored in the refrigerator and particulates appear, wait for the bottle to return to room temperature before mixing. Particulates generally disappear once the 10X bottle has equilibrated. For further instructions, see ihc Wash Buffer IFU. B) Take ihcDirect reagents out of refrigerator approximately 15 minutes before use to allow the reagents to equilibrate. C) Antibodies arrive prediluted and can settle when stored for extended periods of time. Before applying antibody to tissue, invert or gently swirl or shake the bottle 10-20 times.
Incubation temperature too cold. Colder lab temperatures can slow the antibody-antigen reaction and lead to light or no staining.
Run IHC tests between 21-30°C. To accommodate cooler temperatures, users may purchase an ihc Slide Warmer or extend incubation times to accommodate the lab environment;
Use a calibrated thermometer to help verify that room and/or slide warmer temperatures meet the minimum requirements during incubation.
Excess 1X Wash Buffer left on slide can cause dilution of chromogen.
Ensure excess 1X Wash Buffer is tapped off slide and excess fluids are removed from around the tissue and the back of the slide using a Kim Wipe or absorbent towel.
Chromogen incubation times too short.
Use validated incubation time; Increased incubation times may be necessary to that specified or validated by good quality lab procedures.
Counterstain & Coverslip: Hematoxylin, Dehydration/Clearing, & Mounting
Use of the wrong dehydration method or inappropriate mounting media- Non-permanent chromogens can fade if used with alcohols or with non-aqueous mounting mediums.
Ensure your Dehydration/Clearing reagents, chromogen, and mounting media are all compatible. This should be optimized for each tissue type, fixation protocol, and immunostaining intensity desired.
Tissue Preparation: Cryotomy & Fixation
Freeze Artifacts- Freezing tissues too slowly or quickly.
Freezing tissues to slowly or quickly can cause freezing artifact. Use liquid nitrogen spray until you see the OCT start to freeze or place into cryostat for appropriate freezing. Consult your cryostat manufacturer for appropriate temperatures for cutting tissues. Consult other references for methods to properly freeze tissues.
Artifacts, Folds, Compression, and knife lines means the clearance angle is too great and/or dull, nicked blade.
Ensure blade is sharp, clean, and properly angled (~5°).
Incorrect or non-recommended fixative.
Determine best fixative for tissue, generally Acetone or 10% Neutral Buffered Formalin. If signal is too weak, users may try to fix tissues for longer times (e.g. 2-3 minutes).
IHC Staining: Blocking, Antibody, & Chromogen
Blocker incubation too short (or none at all)- unblocked endogenous tissue elements interact non-specifically with primary antibody and/or chromogen.
Ensure that blocking is done for recommended amount of time.
Cross-contamination of Reagents.
Ensure all reagents are stored appropriately based off their respective reagent labels; Should the antibody reagent container appear to be cloudy or precipitates are present in the container, mix gently. If reagents are within specified stability dating and cloudiness persists, consider switching to a new reagent bottle and contact Novodiax Technical Support.
Cross-contamination or Endogenous Peroxidase.
Ensure all reagents are stored appropriately based off their respective reagent labels; Run negative reagent control, omitting antibody and apply chromogen (DAB) only. If staining occurs, then previous stained tissue sections can be confirmed as endogenous peroxidase.Alternatively, tissues can be treated with H2O2 and repeat testing.
Tissue Preparation: Cryotomy & Fixation
Chatter and Cracks in tissue
Insufficient clearance angle and/or inappropriate cutting rate.
Ensure proper clearance angle is used and change rate of cutting.
Counterstain & Coverslip: Hematoxylin, Dehydration/Clearing, & Mounting
Difficulty distinguishing cellular morphology
Hematoxylin incubation time is too long or too short- incubation time is dependent upon the concentration and pathologist preference.
2-45 seconds is typically enough time; Counterstain intensity should be optimized for each tissue type, fixation protocol, and immunostaining intensity desired. For progressive counterstains (Mayer’s, Gill’s), control intensity by varying the incubation time; for regressive counterstains (Harris), adjust staining time to achieve desired intensity.
Oxidation creating crystalline precipitates
Hematoxylin is exposed to light- oxidation creating crystalline precipitates that are observed under the microscope; these crystalline structures can be distracting and, in some instances, make accurate diagnosis difficult.
Ensure hematoxylin is frequently changed and/or filtered per manufacturer’s recommendations.
Cloudy Appearance on slide
No or inadequate dehydration- water is not fully removed creating a hazy appearance to the tissue; cellular morphology can also be affected.
Re-run slides through fresh alcohols and clearing reagent. If problem persists, change out Dehydration/Clearing reagents. Chemical reagents should be exchanged as frequently as dictated by usage. Reagents can become diluted when going from step-to-step. Labs should consider changing reagents every other week at a minimum.
Still have questions?
If you couldn't find what you were looking for and still have questions, don't hesitate to reach out.
Contact Us