Press Releases
Novodiax was voted the “Best Immunoassays Development Company for 2018 & the Most Innovative IHC Technology company” for the Company’s innovative ihcDirect Products.
Select on Press Releases below to learn more!
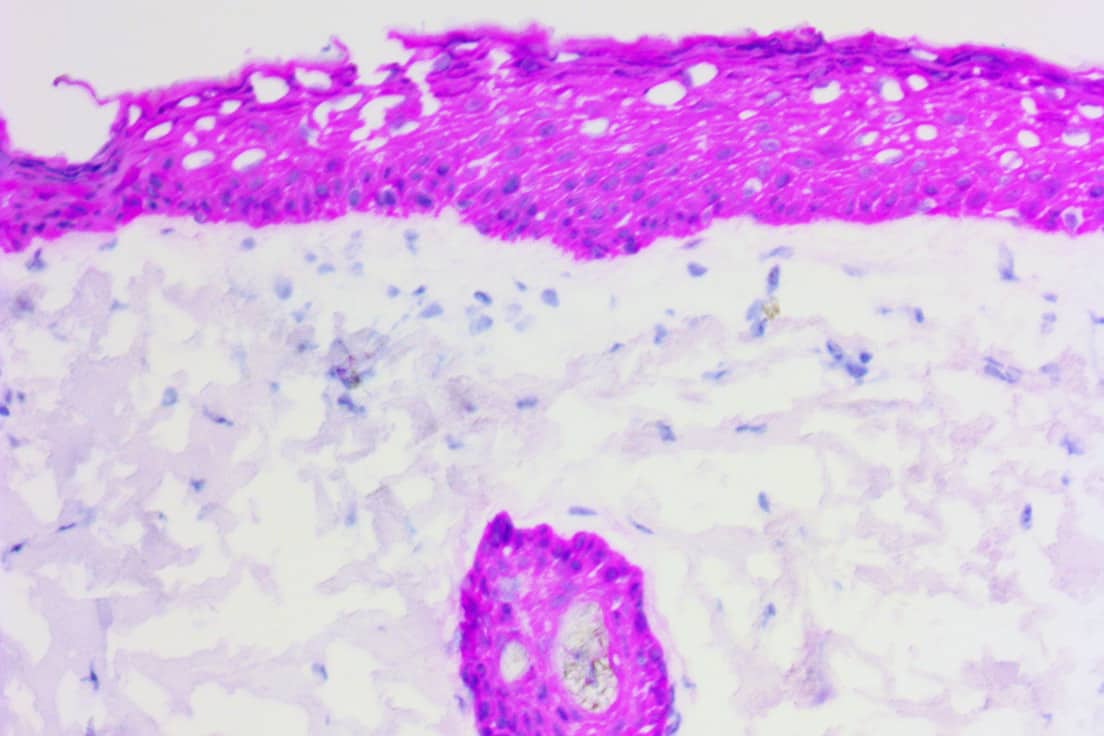
- Rapid ihcDirect® p40 IHC test, for skin and lung cancer testing – November 9th, 2020
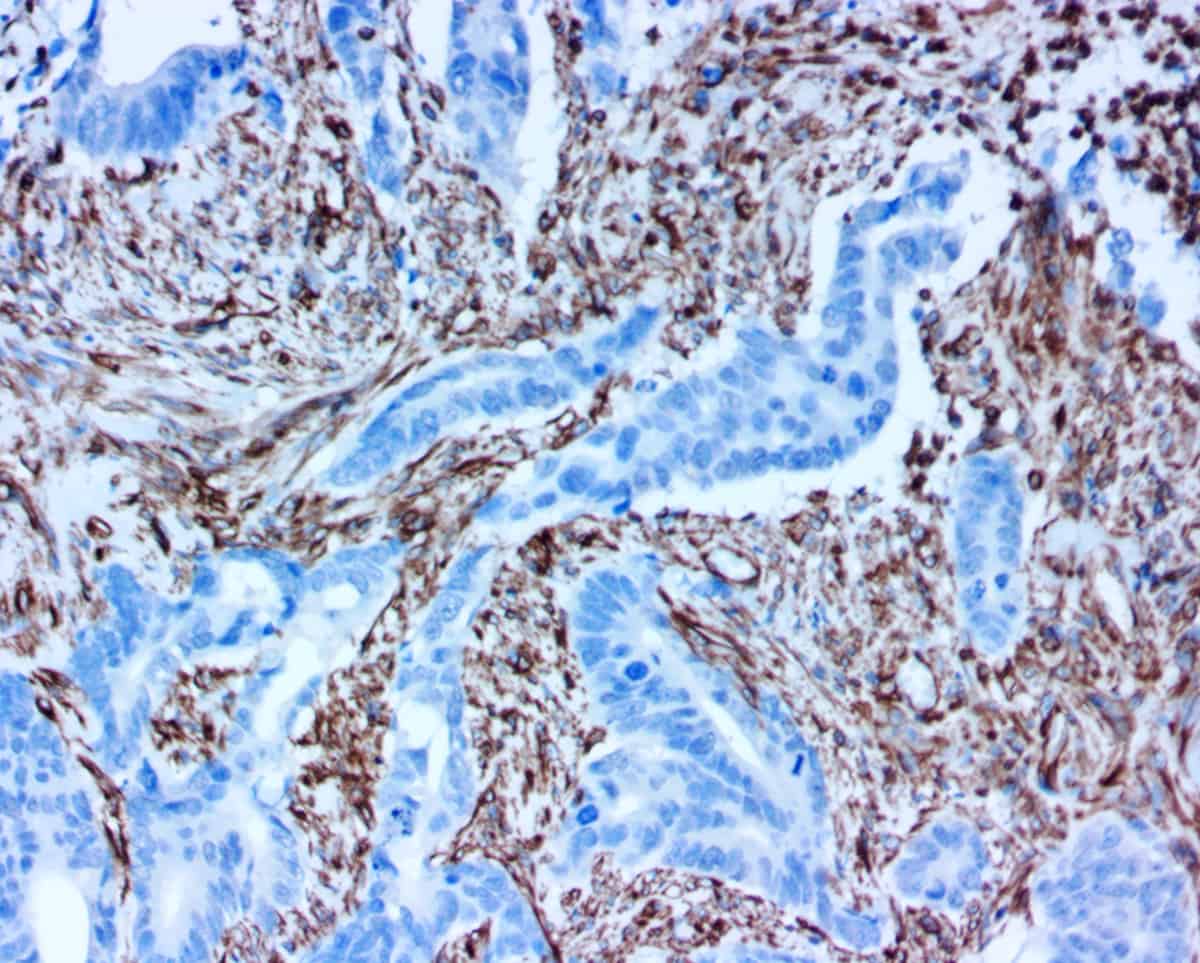
- Novodiax ihcDirect® EpCAM Rapid IHC Test, similar to BerEP4 clone – October 13th, 2020
- ihcDirect® PRAME Rapid IHC Test ready for Use – October 1st, 2020
- ihcDirect® SOX10, first of 5 new IVD IHC assays for Mohs dermatology – July 30th, 2020
- Two new rapid hcDirect® IVD assays, CK19 and CK20 ready for use – July 20th, 2020
- Novodiax Launches HRP Magenta Chromogen for IHC Testing – December 7th, 2019
- Novodiax adds ihcDirect® CD20 and Vimentin antibodies reagents to family of 10-Minute IHC Assays. – December 16th, 2018
- Novodiax Introduces 4 New ihcDirect® Products – August 21st, 2018
- Novodiax Receives Two Awards- Press Release – February 7th, 2018
- Novodiax 10-Minute ihcDirect® Calponin, Podoplanin, CK7, and GFAP Available for IVD Use – April 20th, 2018
- Novodiax 10-Minute ihcDirect® CK8/18 Available for IVD Use – November 7th, 2017
- Novodiax 10-Minute ihcDirect CK5 and Mart-1 (Melan A) Assays Availabel for IVD Use – September 20th, 2017
– – – – – – – – – – – – – – – –